
First Year Lab Number 03 for BS/MS students at IISER Pune
26-30 Aug 2013 in PEB3 Bio-Labs, IISER Pune.
Dr. Chaitanya Athale,
Dr. Gayathri Pananghat,
Dr. Krishanpal Karmodiya,
Dr. Jayeeta Banerjee and
Dr. Shital Ahale
with inputs from Ashwini Keskar and Dr. Neelesh Dahanukar.
Click here to download the PROTOCOL
The most interesting images taken by students will be uploaded, if you send them (with your name and batch) to the coordinator of this module Chaitanya Athale (c a th a le at the rate of i i s er pune etc)

Fig 1: A collage of Arthropods. Copyright Wikipedia.
Phylum Arthropoda: Cu-centred Hemocyanin: Blue when oxygenated. Not all Arthropods show this, for example those belonging to Class Insecta.

Thigh bone in adult humans contains bone marrow which gives rise to the different lineages of the blood cells found in circulation.
Fig 3: Hematopoesis in adult humans begins with cells exiting the bone marrow (Copyright 2001 Terese Winslow, Lydia Kibiuk)
1914: Albert Hustin & Luis Agote demonstrated Sodium Citrate (Na3C6H5O7) as anticoagulant for blood transfusions
Used at concentrations of 3.2% w/v in H2O
Sodium citrate is a mild irritant
First Recorded Blood Storage
Blood bank: 1935 Dr John S. Lundy, Mayo Clinic, USA.
Citrated blood with glucose stored in an “ice box” for 14 days
Patients given citrated blood helped recovery
Central Drugs Standards Control Org., Govt of India
Hepatitis B virus (HBV)
Hepatitis C virus (HCV 3.0)
Human Immunodeficiency viruses, Types 1 and 2 (HIV 1,2)
Human T-Lymphotropic virus (HTLV-I/II)
Syphilis (Treponema pallidum)
Anti-Plasmodium antibody
Peripheral blood (capillary)
Allows identification of blood types
Blood pathogens
Qualitative measure of infection & inflammation by microscopic examination
Relative proportion of blood cells
Basic and gold standard test in diagnostics
Can be preserved
Cheap and easy to do
Label the slide with your name
Clean with a tissue paper to remove grease, industrial coating
Hold the slides only from the edges
Place on a piece of kitchen towel
Unsheath the lancet from protective pack
Swab the finger to be pricked with using 70% Ethanol which has been soaked in piece of cotton (middle or ring-finger)
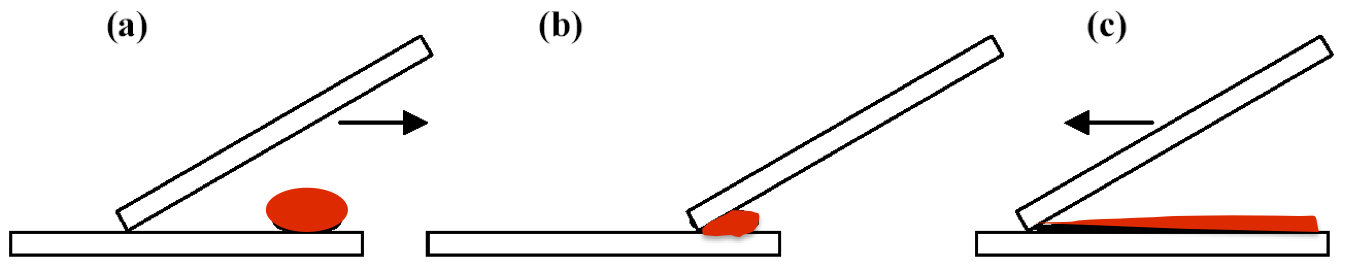
Fig 4: Spreading the blood droplet
3. Discarding Lancet and Swab
Place lancet after use in its original paper cover, and bend it in the middle to ensure no re-use is done
Discard lancet and swab ONLY in designated containers
Use two swabs one before puncturing and one after
Air dry (do not heat) blood smear
Dip 10 s in 99% methanol, let it drip dry
Dip 15 min in concentrated Giemsa stain, let it drip dry
Rinse in water, let it drip dry
Observe under a microscope 10x, 40x (air)
Discard material only in appropriate containers
Clean working area with bleach (or ethanol)
Water artifact: humidity or water in fixative
Crenation: due to slow drying
Smudged cells: blood aging
Leishman Stain (Leishman 1901)
Methylene blue: Basic dye for acidic material (e.g. nucleic acids- DNA, RNA). Colour bluish-purple
Eosin-Y: Acidic dye for basic material. Pinkish colour, usually cytoplasmic
Wright-Giemsa Stain (Wright 1902, Giemsa 1904)
Methylene blue: Basic dye for acidic material (e.g. nucleic acids- DNA, RNA)
Azure B: Basic dye for acidic material (e.g. nucleic acids- DNA, RNA)
Eosin-Y: Acidic dye for basic material. Colour pinkish colour, usually cytoplasmic
Observe different blood cell types
Note and describe colour and morphology as you see
How many morphologically different cell types do you observe? Draw a schematic figure for each.
Differential count: WBCs to RBCs in a given field of view. Compare to textbooks.
Typically 0.3 times the number of RBCs
Prevent bleeding by aggregation in response to injury
Release potent chemotactic factors to aid wound healing
Originate from megakaryocytes
Ultrastructural image in scanning electron microscope (SEM)
White Blood Cell (WBC) Types
| Neutrophil (polymorpho
nuclear leukocytes) |
Basophils | Eosinophils (Granulocytes) | Monocytes | Lymphocytes | |
| Percentage of total WBCs | 40-50% | 0.5-1% | 1-4% | 2-8% | 20-40% |
RBCs do not require staining due to pigmentation. Using citrated-blood the count of RBCs can be made in a calibrated hemocytometer.
High cell densities mean dilution required. Using a Red Blood Cell Dilution pipette we take in 0.5 times volume into the pipette of a lancet-pricked blood droplet. The volume units are arbitrarily selected and the proportionate gradations of 0.1 indicate progression upto 0.5. The remaining pipette volume is made upto 101 (marked beyond the bulb) with 3.2% Sodium Citrate.
A calibrated chamber with grids (XY) and a heavy cover-slip to maintain constant height (Z)- volume constant. The RBC counting region consists of 25 boxes (5x5) bounded by either bold lines (see below left) or triple-lines.
Count in each 4x4 box (figure above, right) the SUM OF RBCs
Use L-rule for cells falling on bounding boxes (vertical line in right box, horizontal line in upper box)
Report your values on the board
Use the mean of 25 boxes for a blood cell count per microlitre
What is the error in counting likely to be? How will you calculate the error? What are the sources of this error?
| Image | Student | Comment |
Mendiratta et al. (2006) Evaluation of different methods for diagnosis of P. falciparum malaria. Ind. J. Med. Microbio.
Molecular Hematology by Provan & Gribben
Clinical Methods: The History, Physical, and Laboratory Examinations.
Marcinek DJ, Bonaventura J, Wittenberg JB, Block BA. (2001) Oxygen affinity and amino acid sequence of myoglobins from endothermic and ectothermic fish. Am J Physiol Regul Integr Comp Physiol. 280(4):R1123-33. [pubmed]